
Enantioselective Synthesis-3
Enantioselective alkylation
Alkylation of aldehydes and ketones
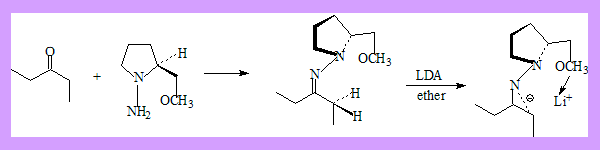
Alkylation of lithiated dimethyl hydrazones forms the basis of asymmetric alkylation of aldehydes and ketones. The following steps are involved.
- Formation of hydrazones of the ketone/aldehydes with a chiral auxiliary group, an amine, ((S)-1-amino-2-(methoxymethyl)-pyrrolidine (SAMP) and its enantiomer (RAMP).
- Formation of an anion using LDA.
- Alkylation.
- Removal of the auxiliary group through hydrolysis of the N-methyl quaternary salt. The following example is an illustration of the procedure.
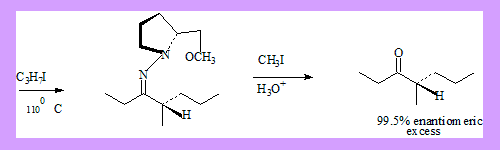
Alkylation of α-amino acids
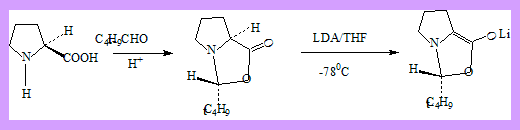
Alkylation of α-amino acids without loss of chirality, or using chiral auxiliary reagents have been effected as follows.
- Proline with pivaldehyde gave a single stereoisomer.
- The product was deprotonated using LDA to the chiral non racemic enolate.
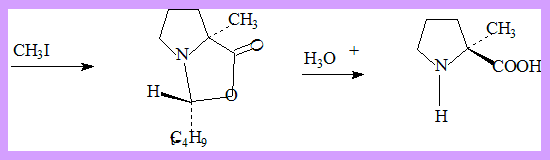
- Attack on the enolate with asymmetric induction by the acetal center takes place
entirely on the same side of the bicyclic system. - The product then readily hydrolyses to optically pure α-methyl proline.
Intra-molecular aldol condensation
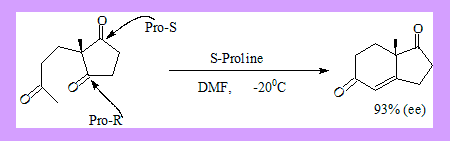
- Intra-molecular aldol condensations in the presence of a chiral base catalyst can proceed with enantio-selectivity. S-Proline catalysed cyclisation of the following ketone proceeds with 93% (ee).
- The two carbonyl groups in the cyclopentane ring are enantiotropic and are distinguished by the chiral catalyst.
Copyrights: 2005 www.chemvista.org All Rights Reserved
The image in the header section is from Wikipedia